
Provision of study materials or patients: Richard Greil, Makoto Tahara, Gilberto de Castro Junior, Amanda Psyrri, Irene Brana, Neus Basté, Prakash Neupane, Åse Bratland, Brett G.M. Access to genetic or exploratory biomarker data requires a detailed, hypothesis-driven statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to an analysis portal so that the requestor can perform the proposed analyses.Ĭonception and design: Barbara Burtness, Denis Soulières, Amanda Psyrri, Joy Ge, Kevin Harrington If the request is declined, it will be communicated to the investigator. There are circumstances that may prevent MSD from sharing requested data, including country-specific or region-specific regulations. Data will be made available for request after product approval in the United States and European Union or after product development is discontinued. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. Applications will be promptly assessed for completeness and policy compliance. The MSD data sharing website (available at ) outlines the process and requirements for submitting a data request.
KEYNOTE 048 TRIAL
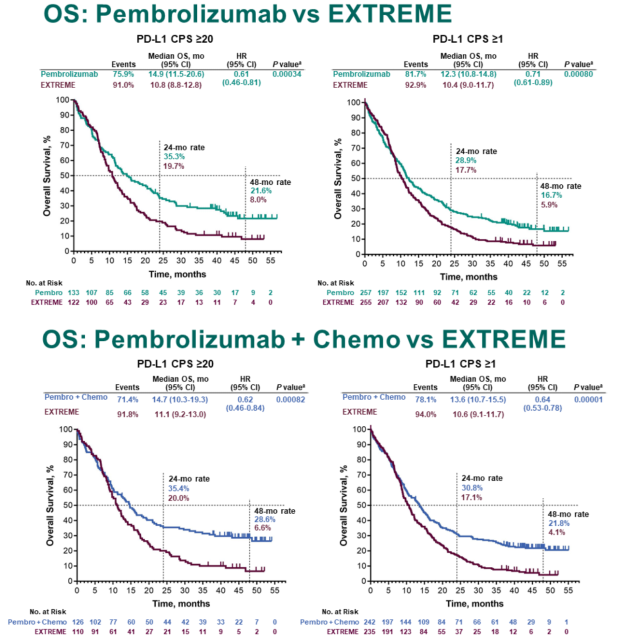
MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. Merck Sharp & Dohme Corp, a subsidiary of Merck & Co, Inc, Kenilworth, NJ (MSD), is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company's clinical trials for the purpose of conducting legitimate scientific research. The results indicate that although PD-L1 expression is useful, additional predictive biomarkers are needed for informing treatment decisions in low PD-L1–expressing recurrent or metastatic head and neck squamous cell carcinoma. These results support previous findings and demonstrate increased efficacy for pembrolizumab or pembrolizumab-chemotherapy with increasing PD-L1 expression.
In the PD-L1 CPS < 1 subgroup, neither pembrolizumab nor pembrolizumab-chemotherapy demonstrated improvement in overall survival compared with cetuximab-chemotherapy. Pembrolizumab and pembrolizumab-chemotherapy demonstrated antitumor activity in the PD-L1 CPS 1-19 subgroup, with pembrolizumab-chemotherapy leading to numerically longer overall survival than cetuximab-chemotherapy. To further characterize the predictive value of PD-L1 expression, efficacy was analyzed in participants with PD-L1 CPS < 1 and CPS 1-19. Efficacy was assessed in participants with a programmed death ligand-1 (PD-L1) combined positive score (CPS) ≥ 20 and CPS ≥ 1 and the total population.
KEYNOTE 048 PLUS
KEYNOTE-048 investigated pembrolizumab monotherapy and pembrolizumab plus chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma.
1Yale University School of Medicine and Yale Cancer Center, New Haven, CTĢPeter MacCallum Cancer Centre and the University of Melbourne, Melbourne, AustraliaģParacelsus Medical University, Salzburg Cancer Research Institute, and Cancer Cluster Salzburg, Salzburg, AustriaĤCentre Hospitalier de l'Université de Montréal, Montreal, Quebec, CanadaĥNational Cancer Center Hospital East, Kashiwa, JapanĦInstituto do Cancer do Estado de Sao Paulo, Sao Paulo, BrazilħNational Kapodistrian University of Athens, Attikon University Hospital, Athens, GreeceĨVall d'Hebron University Hospital, Vall d'Hebron Institute of Oncology, Barcelona, SpainĩUniversity of Kansas Medical Center, Kansas City, KSġ1Medical University of Vienna, Vienna, Austriaġ2Royal Brisbane and Women's Hospital and University of Queensland, Brisbane, Queensland, Australiaġ3Catalan Institute of Oncology, Badalona, Barcelona, Spainġ4Ramathibodi Hospital, Mahidol University, Bangkok, Thailandġ5University Hospital, Zurich, Switzerlandġ6Clinical Oncology Unit, Faculty of Medicine, University of Malaya, Kuala Lumpur, Malaysiaġ8The Institute of Cancer Research and The Royal Marsden NHS Foundation Trust, National Institute for Health Research Biomedical Research Centre, London, United Kingdom


 0 kommentar(er)
0 kommentar(er)
